Lithium-Ion Battery Care Guide
1. Thermal runaway process
1.1 Thermal runaway mechanism
The thermal runaway of lithium-ion batteries is directly triggered by the abnormal increase in their internal temperature. As shown in Figure 1, the thermal runaway of lithium-ion batteries is accompanied by a series of chain reactions. When mechanical abuse occurs, the internal diaphragm ruptures due to deformation of the external structure, and the positive and negative electrodes of the battery are in direct contact, resulting in an internal short circuit, which causes battery abuse. The heat generated by the internal short circuit will cause the battery temperature to rise sharply. When there is no serious internal short circuit, crosstalk between the positive and negative electrodes is also one of the reasons for the abnormal increase in the temperature of high-energy lithium-ion batteries. During the crosstalk process, the oxygen released by the phase change of the positive electrode material reacts with the negative electrode reductive LiCx to generate a large amount of heat. In addition, under low-temperature fast charging conditions, lithium precipitation at the negative electrode of the battery will cause the battery capacity to decay and accelerate battery aging. The precipitated lithium can also react violently with the electrolyte to release heat.
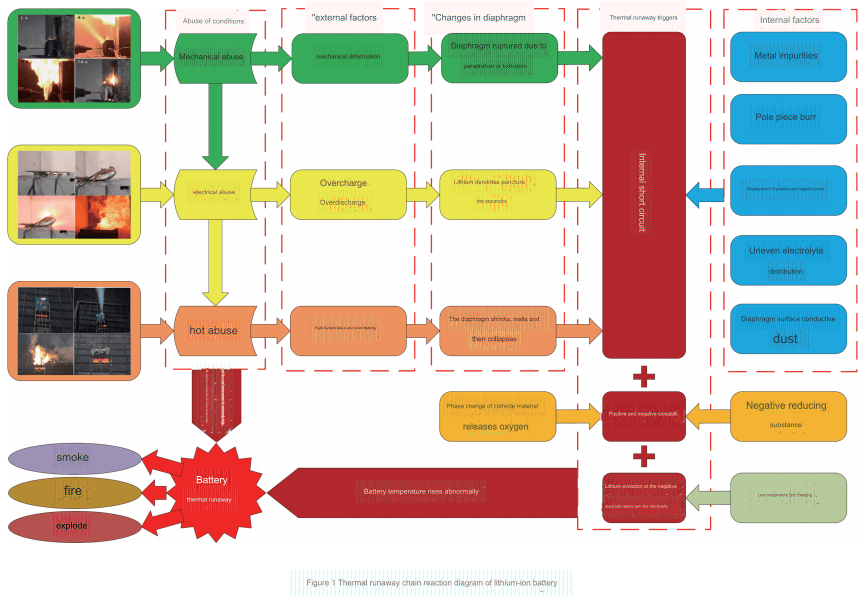
The accumulation of heat will induce thermal abuse, which will lead to a series of exothermic side reactions. Thermal abuse is often the direct cause of thermal runaway of lithium batteries. When the battery temperature reaches the self-heating starting temperature T1, the internal reaction of the battery is relatively slow, the SEI film and electrolyte begin to decompose and are accompanied by slight gas production, the diaphragm morphology does not change significantly, and the interface film on the positive electrode surface thickens. At this time, the battery is in a critical state of thermal runaway. When the battery temperature reaches the thermal runaway initiation temperature T2, the side reactions intensify, the positive electrode surface film further thickens, the internal materials of the battery undergo irreversible changes, side reaction products are generated on the pole piece, and the temperature rises rapidly. As the internal reaction produces gas, the pressure relief valve opens, and the gas containing LiF and combustible organic matter is ejected from the outside of the battery, the carbon-containing material on the positive electrode surface begins to carbonize, the diaphragm is in a molten state, and the side reaction products are evenly attached to the diaphragm. When the battery temperature reaches the highest temperature of thermal runaway T3, the battery diaphragm is completely destroyed, and the positive and negative electrode powders fall off on a large scale.
A. Thermal runaway characteristics
According to different classification methods, lithium-ion batteries are divided into different types. The classification of lithium-ion batteries is shown in Table 1. The thermal runaway process of lithium-ion batteries is complex and changeable. The thermal runaway process of different types of lithium-ion batteries is mainly accompanied by four stages: (a) the exhaust valve opens and the gas is discharged; (b) thermal runaway begins and an open flame is generated; (c) severe thermal runaway occurs and an explosion occurs; (d) the flame is extinguished and the thermal runaway ends.
B. Lithium iron phosphate battery
Due to the strong covalent bond of P=O in the lithium iron phosphate molecule and the octahedral structure of (PO4)3-, lithium iron phosphate batteries have better thermal stability than lithium-ion batteries with other positive electrode materials. Under mechanical load, small-capacity lithium iron phosphate batteries have better resistance to internal short circuit failure. When the mechanical load reaches 8MPa, a local short circuit occurs inside the battery. Under different overcharge conditions, the severity of thermal runaway of 86A·h lithium iron phosphate battery varies with the change of parameters such as charging rate and charging stop time after the exhaust valve is opened. In addition, the heat dissipation of battery module is much lower than that of battery cell, and thermal runaway is more likely to occur under overcharge conditions.
C. Lithium titanate battery
Lithium titanate is a stable spinel structure. As the negative electrode material of lithium-ion battery, it has the characteristics of low theoretical capacity and low conductivity, good thermal stability, good resistance to mechanical and thermal abuse, and poor resistance to electrical abuse. It has been used in some hybrid vehicles. However, the low energy density of lithium titanate battery is still the main factor restricting its further development. Existing studies have shown that lithium titanate battery has good thermal safety under mild electrical abuse, and the uneven SEI film generated on the negative electrode surface is the main cause of thermal runaway of lithium titanate battery. After lithium titanate battery is overcharged, the activity of electrode material will be significantly enhanced, and the thermal stability will decrease rapidly. Under the same thermal abuse conditions, the start time of thermal runaway of battery overcharged to 120% state of charge is shortened by about 1/3 compared with that of battery with 100% state of charge.
1.2 Comparative analysis of thermal runaway characteristics
Table 2 summarizes the thermal runaway characteristic parameters of different types of lithium-ion batteries under different abuse conditions. Mechanical abuse is an external factor that causes thermal runaway of lithium batteries, and usually cannot directly trigger thermal runaway of lithium-ion batteries. Therefore, the summary of abuse conditions in this section mainly focuses on electrical abuse and thermal abuse.
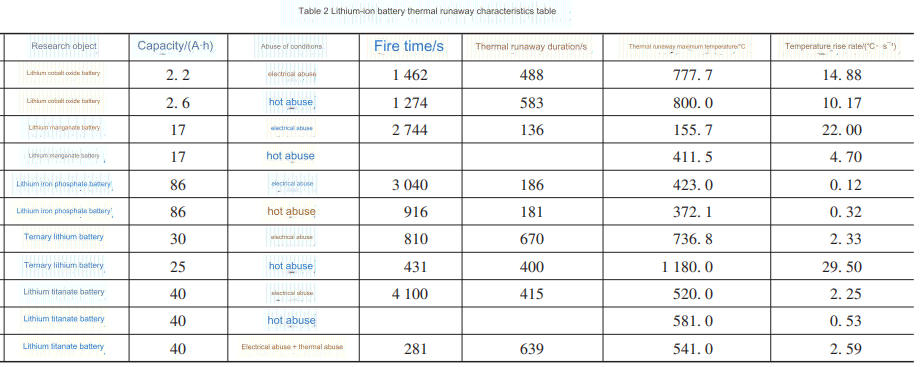
As can be seen from Table 2, when no obvious thermal runaway occurs, the maximum temperature of the battery surface and the temperature rise rate of the battery are both low. Except for lithium iron phosphate batteries, the maximum temperature of other batteries of the same type that have obvious thermal runaway under thermal abuse conditions is generally slightly higher than the maximum temperature under electrical abuse conditions, which is related to the high temperature external conditions when thermal abuse occurs. Different types of lithium-ion batteries have different positive electrode materials, and different chemical reactions occur inside the battery during thermal runaway. Different chemical reaction heat leads to different maximum temperatures of battery thermal runaway. The overcharge resistance of lithium iron phosphate batteries is better than the overheating resistance, while the opposite is true for lithium manganese oxide batteries. This is also due to the large differences in the chemical structure of the positive electrode materials and the electrolyte composition of the two. Lithium cobalt oxide batteries and ternary lithium batteries are relatively more prone to thermal runaway, while lithium titanate batteries are lithium-ion batteries with the best thermal stability. Although different types of lithium-ion batteries have different thermal runaway characteristics under different abuse conditions, collecting and analyzing the thermal runaway characteristic parameters is conducive to reasonably warning and suppressing lithium-ion battery thermal runaway fires based on the characteristic parameters.